
Opportunity
Leverage clinical trial data for actionable, real-time insights

Clinical trial data is fragmented across registries and documents stored in XML, JSON, CSV, HTML, and PDFs, with key facts buried in narrative text. Manual extraction and normalization of eligibility criteria, endpoints, sites, investigators, and amendments slow down decision-making, increase costs, and raise the risk of trial failure. A solution was needed to automate ingestion, harmonization, and analysis while preserving compliance, transparency, and data quality.
Solution
Cut timelines, boost enrollment, improved Diversity, Equity, and Inclusion (DE&I), and safety
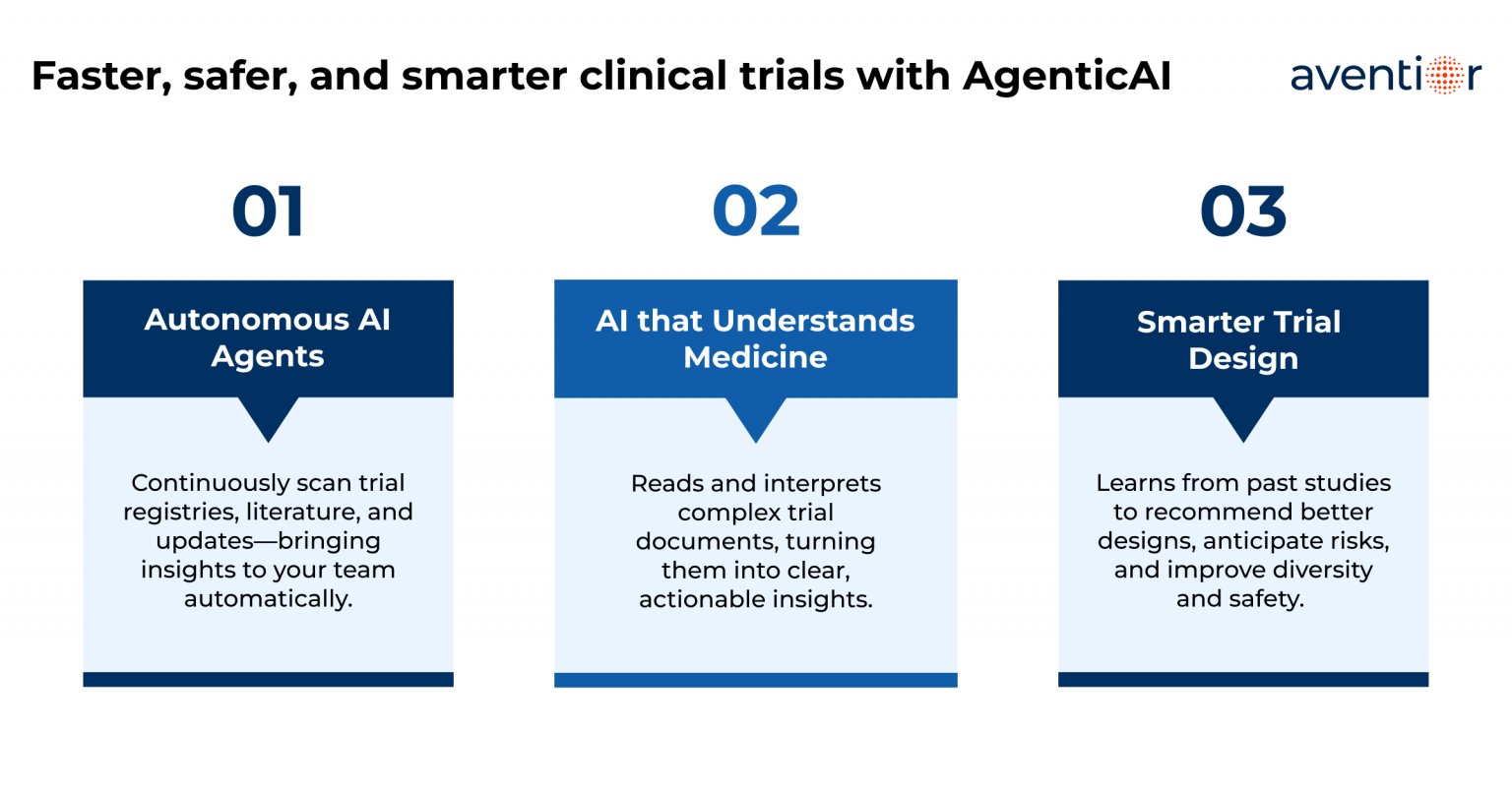
Aventior created an AgenticAI platform that turns scattered trial data into a single, consistent source of truth. The platform automatically reads and organizes information from trial documents, making it searchable and easy to understand. It helps teams design protocols more effectively by simplifying eligibility criteria, refining endpoints, and optimizing visit schedules. Sites and investigators are automatically compared and ranked based on past performance, capacity, and diversity coverage, making it easier to choose the right partners. The system also keeps track of registry updates in real time, flags risks early, and supports compliance by keeping humans in the loop for oversight. In short, the solution cuts through complexity and transforms trial operations into a faster, smarter, and more reliable process.
Action
AgenticAI in Trial Analytics – Key Actions
The system constantly collected and cleaned trial data from different registries and document formats, turning it into a single, reliable source. This made it possible to optimize protocol design and compare ongoing studies in real time. It also highlighted enrollment progress and flagged potential risks early. Eligibility rules were transformed into easy-to-use filters to quickly estimate how many patients could qualify and to spot gaps in representation. Sites were automatically scored and mapped based on their track record, capacity, and diversity coverage. Altogether, the platform created a continuous cycle of intelligence, plan, gather, check, enrich, recommend, and monitor, to deliver timely, actionable insights.
Impact
- Protocol development timelines reduced by 40–60%, with higher regulatory alignment.
- Site and investigator selection improvements driving 25–35% better enrollment performance and stronger DE&I coverage.
- 30–50% fewer protocol amendments through upfront endpoint and criteria optimization.
- Early risk detection 3–6 months sooner, plus faster safety signal identification by 40–60%.
- Stronger regulatory readiness through lineage-aware evidence and transparent rationales.



 +1 (617) 221-5900
+1 (617) 221-5900
 Follow Us
Follow Us
